DNA cloning and modification (SLIC)
“Failure” happens all the time in science, that’s just a fact of life. I remember during new student orientation for graduate school, a professor said “If 90% of your experiments fail, you are making good progress and will graduate on time”, which is true. Shocking on the surface, but it makes sense if you define fail as “didn’t produce an interpretable result” instead of “didn’t give the result YOU wanted”.
In science, you want to constantly be pushing our understanding/knowledge to new limits, which means asking new, tough questions and designing experiments around your ideas. Cool science is a double-edged sword, however; truly original experiments require a lot of effort to get working (optimization). This is what I’ve been spending a lot of time doing these past few weeks: optimizing a new way to clone and mutate DNA in our lab known as Sequence and Ligation Independent Cloning (SLIC).
Traditional cloning/mutation experiments can be tedious. You need to run several reactions for hours at a time (sometimes overnight). There are quick mutation kits, but these are limited in what they can do (several amino acid mutations at a time vs adding a piece of another protein). The beauty of SLIC is that it uses the laws of physics and biology to do the heavy lifting for you. Another way to think of these experiments is like changing the color the band on an old Fitbit vs changing it out a new one; the new Fitbit makes it relatively quick and easy to change out the bands, whereas the original Fitbit requires special tools and a little skill.
Figure 1
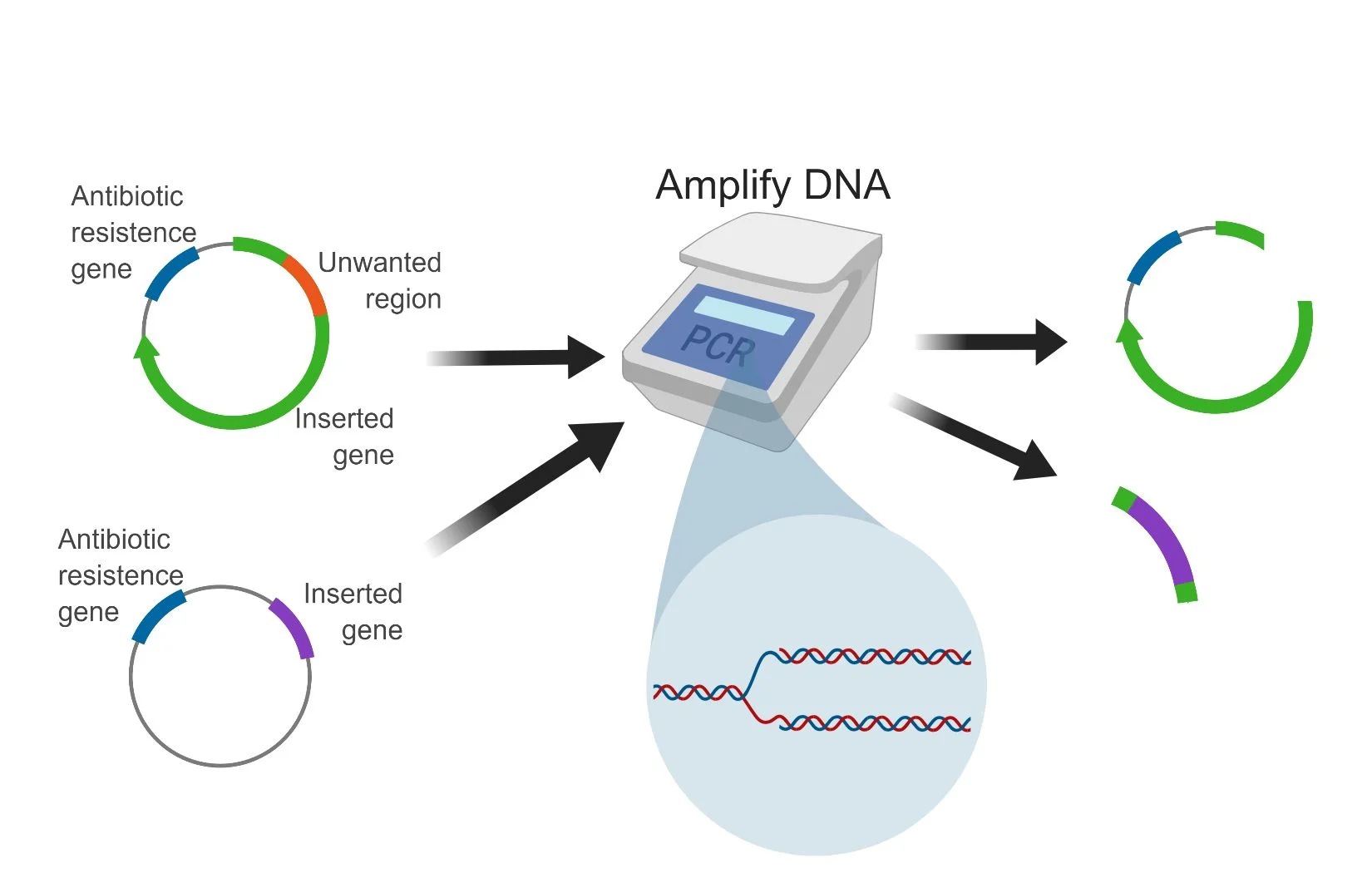
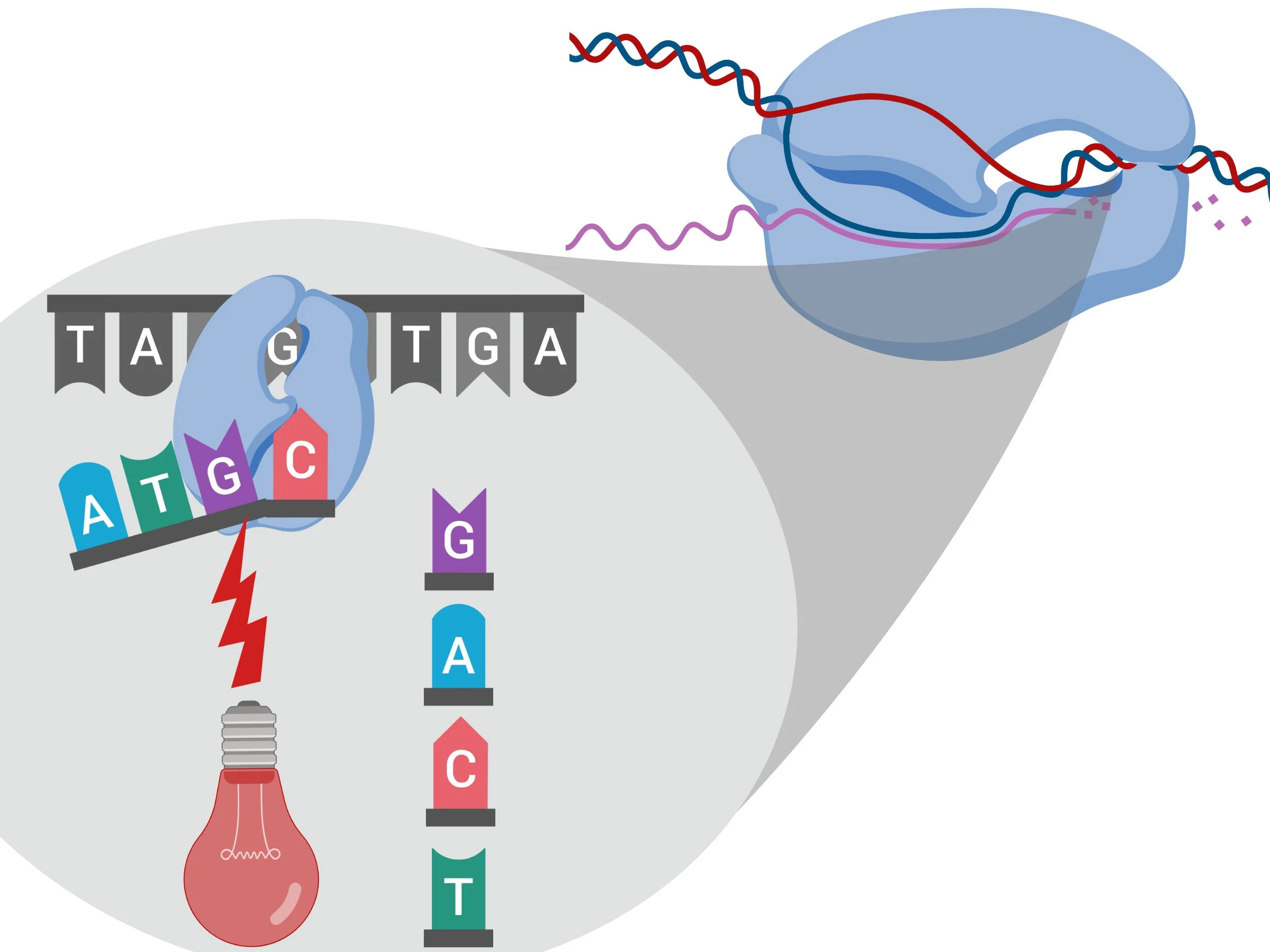
In SLIC, you start by performing two polymerase chain reaction (PCR) experiments, which amplifies the specific regions of DNA I’m interested in (see figure 1). In this experiment, I’m amplifying circular DNA called plasmids. However, I don’t just amplify the DNA, I add DNA base pairs to one end that are complementary to the other. I then clean up the reaction, and then start a new reaction, where I use an enzyme (see figure 2) to “chew back” pieces of the double stranded DNA, making the ends of each DNA piece single stranded (see figure 3). Since they’re complementary to one another, they have the potential to stick together, although there are no chemical bonds, and this interaction isn’t strong enough to hold them together permanently.
Figure 2
Figure 3
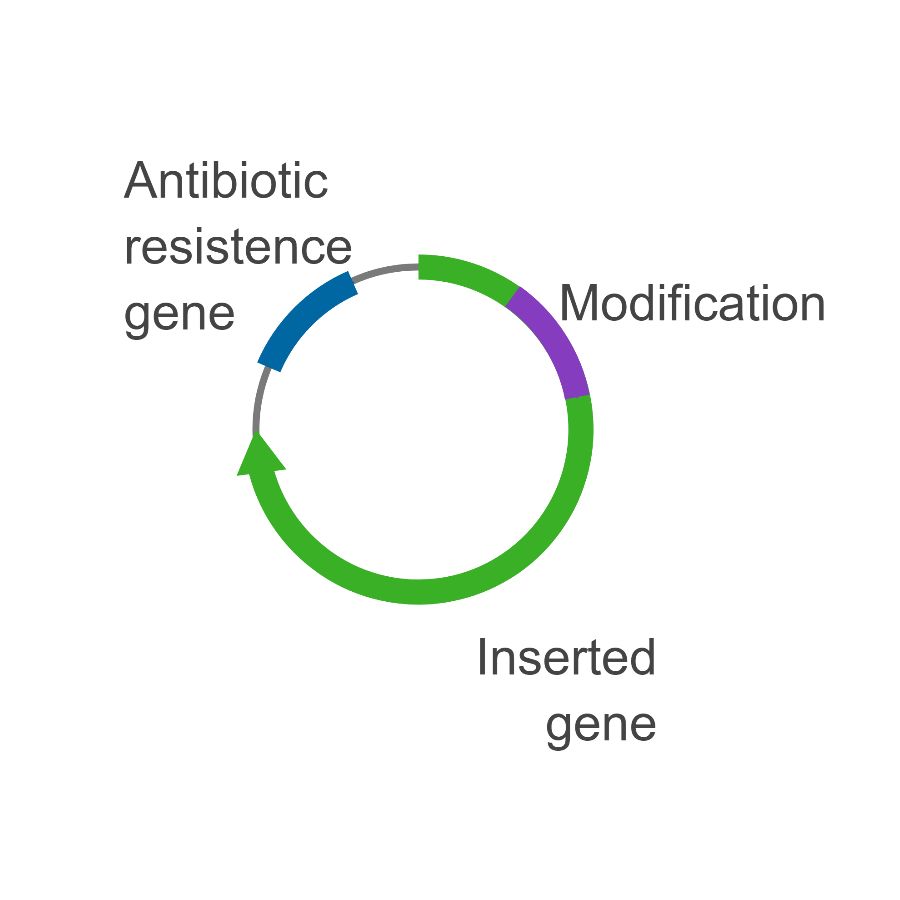
This is where bacteria comes in: I put mix the two pieces of DNA with the bacteria specifically designed to take up DNA. The bacteria thinks the DNA is its own, and tries to “Fix” the broken DNA using enzymes that glue the DNA pieces together, i.e. ligation (see figure 4).
Figure 4
To make sure I have the bacteria containing my product, I grow them in the presence of antibiotics, so that the only way bacteria can survive is if they have the complete (circular), ligated plasmid, since the plasmid has a gene that allows it to grow despite the antibiotic (see figure 5).
Figure 5
It’s a cool, fast way to modify DNA, in theory. In practice, you have to figure out how much DNA to use, how long you should be doing the “chew back” reaction for (and at what temperature). That’s where I’m at right now.
To design my experiments, I’ve been reading through several sources that describe their way of doing SLIC, including the original publication. Also, Addgene.con has been a huge help! Its a website that lets scientists deposit plasmids that they make, which other can then request and use. They even include videos and guides on how to do molecular biology experiments.
LB-Agar/Ampicillin plate before (left) and after letting bacteria grow overnight.
I’m at the part where I (hopefully) purify the new product today! I started the experiment over the weekend, and put the DNA into bacteria Sunday. Yesterday I saw bacterial colonies, and I picked out a few to grown overnight.
I’ve been working on getting this experiment to work since New Year’s Eve, and have been designing it since November. Hopefully, I’ll have some cool results to show soon!