Ten Clamp Commandments (Part 1)
I learned patch-clamp electrophysiology during the pandemic. No flex, no cap, just facts. This is some of the wisdom I gained from joining a neurophysiology laboratory as someone who’s never taken a neuroscience class before. Title inspired by the great Christopher Wallace; cover art inspired by the indescribable feeling of patch-clamp electrophysiology.
Disclaimer
The lab I worked in is probably not like your lab and I recorded mostly sIPSCs from Sprague-Dawley rat CeM bains. This is not The Axon Guide or Areles Mollerman’s book “Patch Clamping: An Introductory Guide To Patch Clamp Electrophysiology”. I suggest you read those as guides for learning patch principles, and read this when (not if) you’re having a bad day at the rig.
Know what you must (and hopefully more)
You can understand 100% of the theory behind electricity and magnetism and still have a terrible time doing electrophysiology. However, the converse can also be true; you can struggle with the E&M principals that make electrophysiology possible yet still produce high quality data. Extreme but not improbable scenarios.
Like all of science, the more you know the better, but if I’m being honest, you only need to know enough to do good science. That means different things to different people, and while knowing everything is desirable, it’s probably not required. Don’t confuse current and voltage, and know what the different clamp protocols tell you. You will have plenty of time to learn the theory while you’re incubating your brain slices with drugs.
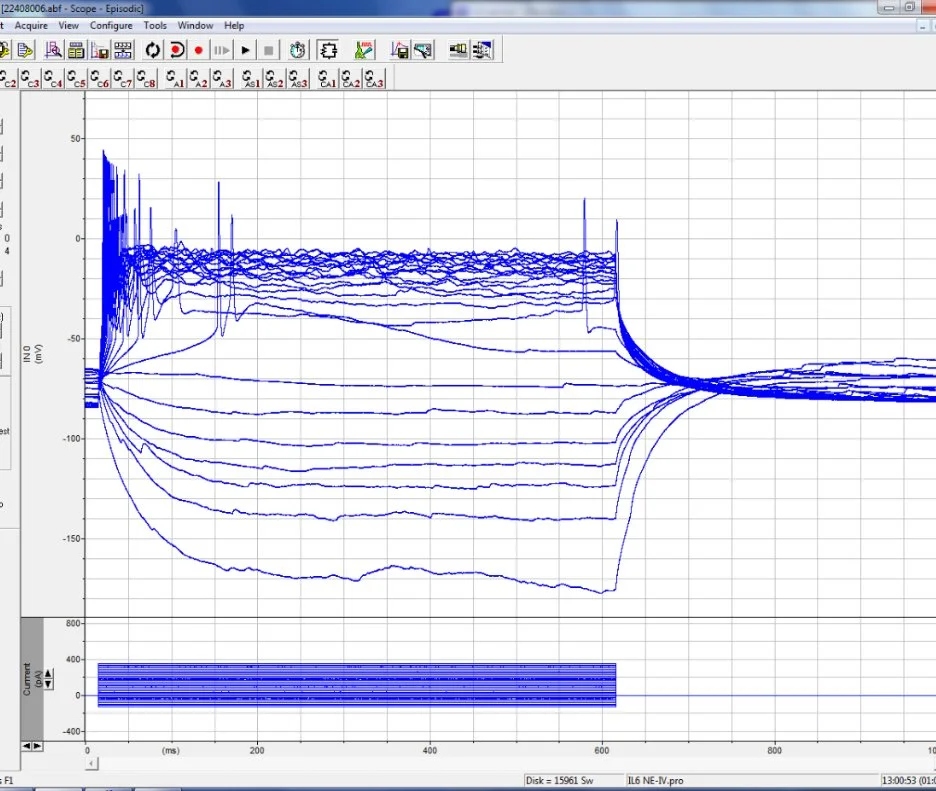
An IV experiment. Huge potential (pun) for hardcore neuroscience. For some regions, it can be extremely informative. In my case, a low-risk experiment to run just before my main sIPSC recordings.
Make it easy
Identifying the different brain regions was a big challenge for me because I have never taken a class in neuroscience or neuroanatomy. The early days of the pandemic with social distancing made things even more difficult because patch clamp electrophysiology is a ridiculous technique. You’re supposed to record the electrical properties of neurons from very thin slices of rat brain tissue. You’re super careful to keep them alive and not deprived of oxygen, but you move them around with a paint brush from hobby lobby. The rig to run experiments costs an easy $150k, but if you or someone in the lab plugs something into the wrong electrical outlet, your data might become unusable. Most electrophysiologists are experts at mouth-pipetting, which is super illegal in science. Our conversations with the safety officers at Scripps were amazing: “I know we’re in the middle of the pandemic but no, we really can’t wear masks right now because we have to mouth-pipette. No, we aren’t eating in the lab, the sucrose slushy is attracting ants. No, we use that spoon for rat brain experiments. Why are you looking at us like that?”
A central amygdala slice from a rat. At the time, it seemed like a cruel rorschach test for my scientific career. Today, I’m like “Yes, the BLA is clearly visible and the tracts show great definition. Wonderful slice.”
Anyway, it’s possible to learn as you go, just make sure to take it easy on yourself. I had a PDF copy of the rat brain atlas on all my computers for reference. The digital microscopes were also a lifesaver, because in my opinion, learning to pick up a rat brain with a paint brush is weird and unnatural but it has to be done. No lie, in the beginning it took me like 5 minutes to mount the brain slice into the recording chamber because on my rig and in my hands, it loved to float away into the vacuum line (I created a vacuum switch to get around this). After fighting the slice to stay in place (without smashing/scratching it!), it freaking sucks be told that the slice was too ventral or dorsal. Also, I took screenshots of the CeM and asked my lab mates to draw the region they would record from using MS word. Lastly, I had sticky notes with drug dilution calculations (25, 50, and 100 mL final volumes) because if you’re fighting the cell to stay open, you literally don’t have time to think, you can only do.
Mindstate: strategically resilient
Brain slices are surprisingly resilient. Just because your aCSF stopped bubbling (for 30 minutes) doesn’t mean your slice is totally dead. Tubing gets clogged, valves don’t open, things happen. Just remember to constantly keep an eye on your rig.
Science is hard and requires grit cuz experiments fail. Electrophysiologists are a different breed when it comes to resilience though. This is because electrophysiology experiments can be the lowest throughput imaginable (one cell/animal brain per data point each day). If that wasn’t painful enough, these experiments can fail. These failures can be for high level reasons, such as you being the first person to study your thing so you have a lot to figure out. However, most of the time, experiments can/will fail for exceptionally stupid reasons, such as the water/osmometer/pH meter/carbogen/waterbath/tubing/pipette in your lab/rig going bad intermittently. Or your rig flooded. Or maybe a random noise appears one day and is gone the next with no explanation. As a scientist, the worst failures of all come from unknown reasons, which is a weird grey zone between the two. This is why electrophysiologists tend to be superstitious; it’s a survival mechanism but also, you could 1000% chase ghosts if you don’t learn to triage and move on.
Thinking quickly and luck
If you’re lucky, you can avoid a stupid failure. For example, you test some leftover aCSF and you find that works better than the fresh/new aCSF, or maybe you have an manometer connected to your tubing line so you can detect a leak/crack in your vacuum. Maybe the first thing you try to troubleshoot turns out to be the problem. Sometimes you can get a sense of what’s going on depending on the issue, but when you have a brain slice dying by the hour, sometimes you have to throw solutions at the problem as quickly as possible. Sometimes you just picked up a dead slice. Literally: the slice you picked is the only one with dead neurons and the other slices are super healthy. Maybe the cutting procedure is not gentle enough, or a piece of ice hit your vibratome blade, which killed one side of the slice. I’m just speculating because this happens more often than you think and you can’t always figure out the problem. If slices are limited and/or you don’t want to toss the slice, flip the slice over before you throw it away. Maybe you will find that the cells are only dead on one side. Of course, you gotta judge on whether that’s ok for you to do for your experiments. On that note, don’t be afraid to throw away slices if you have them. Since I knew I had 3-4 slices to work with each day, I would never spend more than 90 minutes on a slice.
That said, you can’t definitively increase luck. However, you can reduce the probability of stupid failures, so I came up with a maintenance routine for my rig where I’d regrease my gaskets and chloridize my electrodes each week. Usually, you can visibly tell when you need to do maintenance, but it was easier for me to just always do this the first day of experiments (usually 8am on Monday mornings). I also kept my rig simple and set/warm everything up about 45 minutes before I’d begin recording. If you have a weird issue with your camera or with pClamp, sometimes a restart fixes everything. It’s much worse when these issues arise in the middle of an experiment.
On the topic of internal solutions, I will say that from a practical standpoint, neurons that do not look absolutely beautiful may prefer a different osmolarity. You should not make drastic changes to your protocol because you think your slices are unhealthy, but since we are generally working within ranges, you may find that using solutions in the lower/higher range makes things easier/harder depending in how your cells look. I found that while my 50 mLs of internal solution took an incredible 2 hours to make (thank you HEPES), it was never the problem, so I could freeze single-use aliquots without worrying. Other people preferred making 2 mLs of internal solution fresh each day, which is also valid because we were using the same animal slices, reagents, equipment, recipes, and ranges.